
The tabulated data are examples of a few possible values. Millikan’s experiment measured the charge of individual oil drops. The term “electron” was coined in 1891 by Irish physicist George Stoney, from “ electric i on.”įigure 2.
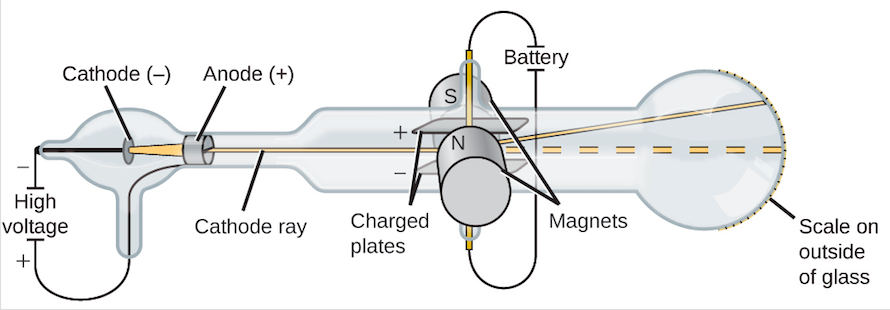
Although controversial at the time, Thomson’s idea was gradually accepted, and his cathode ray particle is what we now call an electron, a negatively charged, subatomic particle with a mass more than one thousand-times less that of an atom. (credit a: modification of work by Nobel Foundation credit b: modification of work by Eugen Nesper credit c: modification of work by “Kurzon”/Wikimedia Commons)īased on his observations, here is what Thomson proposed and why: The particles are attracted by positive (+) charges and repelled by negative (-) charges, so they must be negatively charged (like charges repel and unlike charges attract) they are less massive than atoms and indistinguishable, regardless of the source material, so they must be fundamental, subatomic constituents of all atoms. Simultaneous deflections by applied electric and magnetic fields permitted Thomson to calculate the mass-to-charge ratio of the particles composing the cathode ray. (c) In the cathode ray, the beam (shown in yellow) comes from the cathode and is accelerated past the anode toward a fluorescent scale at the end of the tube. (b) This is an early cathode ray tube, invented in 1897 by Ferdinand Braun. Thomson produced a visible beam in a cathode ray tube. The results of these measurements indicated that these particles were much lighter than atoms (Figure 1).įigure 1. In similar experiments, the ray was simultaneously deflected by an applied magnetic field, and measurements of the extent of deflection and the magnetic field strength allowed Thomson to calculate the charge-to-mass ratio of the cathode ray particles. This beam was deflected toward the positive charge and away from the negative charge, and was produced in the same way with identical properties when different metals were used for the electrodes. When high voltage was applied across the electrodes, a visible beam called a cathode ray appeared between them. This apparatus consisted of a sealed glass tube from which almost all the air had been removed the tube contained two metal electrodes. If matter were composed of atoms, what were atoms composed of? Were they the smallest particles, or was there something smaller? In the late 1800s, a number of scientists interested in questions like these investigated the electrical discharges that could be produced in low-pressure gases, with the most significant discovery made by English physicist J. Atomic Theory after the Nineteenth Century While the historical persons and dates behind these experiments can be quite interesting, it is most important to understand the concepts resulting from their work. Here, we will discuss some of those key developments, with an emphasis on application of the scientific method, as well as understanding how the experimental evidence was analyzed. Much of this came from the results of several seminal experiments that revealed the details of the internal structure of atoms. In the two centuries since Dalton developed his ideas, scientists have made significant progress in furthering our understanding of atomic theory. Define isotopes and give examples for several elements.Describe the three subatomic particles that compose atoms.

Summarize and interpret the results of the experiments of Thomson, Millikan, and Rutherford.Outline milestones in the development of modern atomic theory.


 0 kommentar(er)
0 kommentar(er)
